Homework 4: Risk
Homework objectives
Use these problems to practice Risk analysis, including, where appropriate, ARR, RRR, Odds, and NNT (or NNH)
Homework 4 expectations
Read through the entire homework before starting to answer a question. You are expected to have read the chapter and to have completed preceding homework. Answers are provided to numbered odd problems — turn in your work for even numbered problems.
How to work this homework
You may work together, but each of your must turn in your own report. Don’t “plagiarize” from each other. Do include in your report who you worked with.
What to turn in: A pdf file containing your R code, statistical results, and your answer to the questions. Use of RMarkdown recommended; however copy/paste into a word document is also acceptable.
Submit your work to CANVAS. Obey proper file naming formats.
Resources for this homework
Mike’s Biostatistics Book: Chapter 7
Mike’s Workbook for Biostatistics: A quick look at R and R Commander, Part01 – Part10 and previous homework pages presented in this workbook.
RcmdrPlugin.EBM can help with some of the calculations. See Chapter 7.4 in Mike’s Biostatistics Book.
Additional R commands and or code provided below.
Questions
1. Sensitivity of a test is defined as
- False Positive Rate
- True Positive Rate
- False Negative Rate
- True Negative Rate
2. Specificity of a test is defined as
- False Positive Rate
- True Positive Rate
- False Negative Rate
- True Negative Rate
3. In thinking about the results of a test of a null hypothesis, Type I error rate is equivalent to
- False Positive Rate
- True Positive Rate
- False Negative Rate
- True Negative Rate
4. What is the primary outcome of screening (e.g., mammogram for breast cancer, fecal immunochemical test for colorectal cancer), for evidence of cancer?
- Five-year survival rate after diagnosis
- Mortality rate after diagnosis
- Presence of cancer
- Positive or negative screening result
5. Both odds and relative risk are used to quantify association between an exposure and an outcome. The two are not identical, however. When the outcome is rare, odds and relative risk will yield similar conclusions. When outcome is common, results of odds and relative risk calculations may lead to drastically different conclusions. For each of the three designs listed below (A,B,C), identify whether odds, relative risk, or both can be used
- Case-control retrospective study
- Cohort prospective study
- Randomized control trial
Hint: Which type of study is the number of exposed subjects unknown?
6. Chromosome mutations are surprisingly frequent in human reproduction. The estimated distribution of chromosome mutations among all human conceptions that develop sufficiently to implant in the uterus is 8%. Among live births, 0.61% have chromosomal abnormalities. The most accurate test for chromosomal mutations requires amniocentesis. Amniocentesis carries a risk of miscarriage, about 1 in 200 (about 200K procedures annually, per Mayo Clinic; a recent systematic review places the risk at about 15 in 2000 procedures, per Akolekar et al 2015). What is the chance that a pregnancy that is miscarried due to amniocentesis actually has a chromosome abnormality?
7. Detection of Down syndrome requires acquisition of fetal cells, and the standard method is amniocentesis. Amniocentesis — like any invasive medical procedure — carries a risk of miscarriage. How risky? Difficult to know for any particular hospital or doctor, but the national rate is about 1 in 200 procedures (about 200K procedures annually, per Mayo Clinic; a recent systematic review places the risk at about 15 in 2000 procedures, per Akolekar et al 2015). Risk of chromosomal abnormalities increases with maternal age. Thus, decision to be tested must involve a weighing of risks: Risk of chromosomal abnormalities to the fetus and increasing maternal age vs. risk of amniocentesis and spontaneous miscarriage. A graph is shown below (Fig. 1, data from CDC), which characterizes the risk to the fetus by maternal age and in the context of live births by maternal age category.
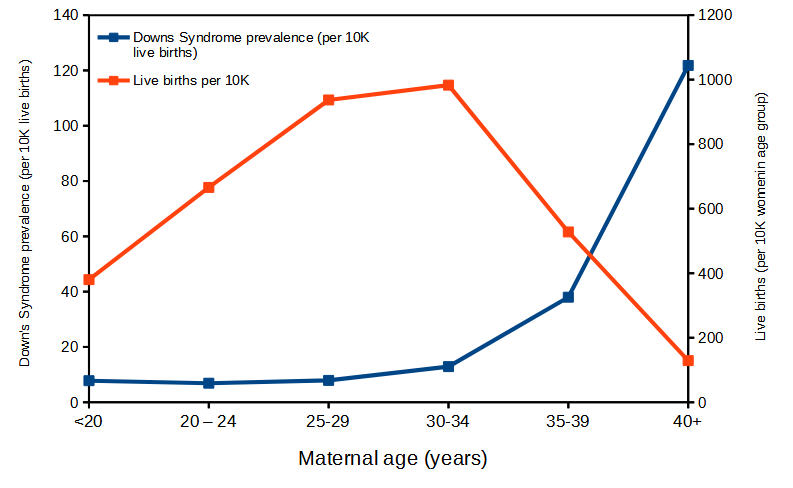
Figure 1. Live births (red) and Down’s Syndrome (blue) per 10,000 women by maternal age groups. In 2010, about 4 million live births of an estimated 6.2 million pregnancies. Non live births include 1.1 million induced abortions and 1.05 million fetal losses. Data from CDC
At what maternal age is the number needed to harm NNH approximately equal to 100? To 200? (NNH is the same concept as NNT, but instead of “treat” we write “harm” and infer risk of spontaneous miscarriage due to amniocentesis).
8. Women’s Health Initiative study investigated benefits and risks of replacement estrogen therapy in women (data derived from Figure 2, Manson et al 2013). The incidence of breast cancer in the treatment group (conjugated equine estrogens and medroxyprogesterone acetate) was 206 per 8506 women. The incidence in the placebo group was 155 per 8102 women. What was the Relative Risk Reduction, Absolute Risk Reduction, NNT, and Odds ratio?
9. Cases were men and women diagnosed with oral–oesophageal cancer in relation to coffee drinking habits. Data were from a cohort, prospective study of Norwegian men and women between age 40 – 45 followed for about a decade (data derived from Table 1 and Table 2, Tverdal et al 2011 – numbers reported in this table were not adjusted for possible confounding variables — see Tverdal et al for complete analysis).
|
|
Oral–oesophageal cancer | ||
|
Cups of coffee per day |
Cases | Noncases |
Total |
| < 1 cup | 35 | 41,419 |
41,454 |
|
1 or more cups |
415 |
347,755 |
348,170 |
| Total | 450 | 389,174 | 389,624 |
Does coffee drinking increase risk of oral cancer? Calculate the Relative Risk Reduction (and Odds ratio), Absolute Risk Reduction, and NNH.
10. The sensitivity of the fecal occult blood test (FOBT) is reported to be 0.68. What is the False Negative Rate?
11. The specificity of the fecal occult blood test (FOBT) is reported to be 0.98. What is the False Positive Rate?
12. For men in the United States between 50 and 54 years of age, the rate of colon cancer is 61 per 100,000. If the false negative rate of the fecal occult blood test (FOBT) is 10%, how many persons who have colon cancer will test negative?
13. For men in the United States between 50 and 54 years of age, the rate of colon cancer is 61 per 100,000. If the false positive rate of the fecal occult blood test (FOBT) is 10%, how many persons who do not have colon cancer will test positive?
14. A study was conducted to see if mammograms reduced mortality
| Deaths/1000 women | |
| No mammogram | 4 |
| Mammogram | 3 |
What is the RRR?
15. A study was conducted to see if mammograms reduced mortality
| Deaths/1000 women | |
| No mammogram | 4 |
| Mammogram | 3 |
What was the NNT?
16. Does supplemental Vitamin C decrease risk of stroke in Type II diabetic women? A study conducted on 1,923 women, a total of 57 women had a stroke, 14 in the normal Vitamin C level and 32 in the high Vitamin C level. What is the NNT between normal and high supplemental Vitamin C groups?
17. During the COVID-19 pandemic several treatments were pushed by the public. For example, Dwayne “The Rock” Johnson pleaded for COVID-19 survivors to donate their plasma (Facebook video). On 23 August 2020, COVID-19 convalescent (“survivor’s”) plasma was approved for emergency use by FDA to treat patients with COVID-19 (FDA link). Researchers (Korley et al 2021) recruited 511 patients who presented to emergency departments with mild COVID-19 symptoms during first week after infection. Patients were randomly assigned to receive convalescent plasma treatment (n=257) or placebo treatment (n=254) to test whether survivor’s plasma prevented progression to severe COVID-19 illness. Disease progressed in 77 patients (30%) in the plasma treated group compared to 81 patients (31.9%) in the placebo group. Calculate RRR, the odds ratio, and NNT. Is “survivor’s” plasma a promising treatment?
References
Akolekar, R., Beta, J., Picciarelli, G., Ogilvie, C., & D’Antonio, F. (2015). Procedure‐related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta‐analysis. Ultrasound in Obstetrics & Gynecology, 45(1), 16-26.
Centers for Disease Control and Prevention. (2021, August 3). FastStats – Births and Natality. Centers for Disease Control and Prevention. Retrieved October 12, 2021, from https://www.cdc.gov/nchs/fastats/births.htm
Gigerenzer, G. (2015). Calculated risks: How to know when numbers deceive you. Simon and Schuster.
Korley, F. K., Durkalski-Mauldin, V., Yeatts, S. D., Schulman, K., Davenport, R. D., Dumont, L. J., … & Callaway, C. W. (2021). Early convalescent plasma for high-risk outpatients with Covid-19. New England Journal of Medicine.
Manson, J. E., Chlebowski, D. R. T., Stefanick, M. L., Aragaki, M. A. K., Rossouw, J. E., Prentice, R. L., … & Wallace, R. B. (2013). The Women’s Health Initiative hormone therapy trials: update and overview of health outcomes during the intervention and post-stopping phases. JAMA: the journal of the American Medical Association, 310(13), 1353.
Martin, J. A., Hamilton, B. E., Osterman, M. J., & Driscoll, A. K. (2021). Births: Final Data for 2019. National Vital Statistics Reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 70(2), 1-51.
Tverdal, A., Hjellvik, V., & Selmer, R. (2011). Coffee intake and oral–oesophageal cancer: follow-up of 389 624 Norwegian men and women 40–45 years. British journal of cancer, 105(1), 157-161.
/MD